Abstract
Introduction: c-myc is an oncogene implicated in the initiation and progression of multiple hematologic malignancies. Characteristic translocations of the c-myc gene with the immunoglobulin heavy chain gene (in Burkitt lymphoma and "double-hit" non-Hodgkin lymphoma) and with the T cell receptor gene (in T cell acute lymphoblastic leukemia, T-ALL) lead to constitutive expression of the c-myc transcription factor, ultimately driving dysregulated proliferation (Boxer & Dang, Oncogene 2001; 20(40):5595-5610). Strategies for inhibition of c-myc remain an area of active research; however, to date there are no approved myc-targeted agents within the cancer armamentarium, and cytotoxic chemotherapy remains the standard of care for c-myc-driven lymphoid malignancies. Protein phosphatase 2A (PP2A) is a serine/threonine phosphatase tumor suppressor protein that contributes to c-myc degradation through both direct dephosphorylation of an identified phosphodegron as well as by indirect mechanisms (Farrell & Sears, Cold Spring Harbor Perspect Med 2014; 4(3):1-15). Our laboratory has developed a series of first in class, orally bioavailable small molecule activators of PP2A (SMAPs) by derivatizing tricyclic antipsychotics to maximize antiproliferative activity and minimize psychotropic properties (Sangodkar et al., J Clin Invest 2017;127(6):2081-2090). We hypothesized that activation of PP2A with SMAPs would lead to c-myc degradation and tumor growth inhibition in models of c-myc-driven lymphoid malignancies.
Methods: We investigated the effects of SMAP treatment on c-myc content, c-myc half-life (t1/2), cell viability, and xenograft tumor growth in Burkitt lymphoma (BL) and T-ALL cell lines with wild type and mutant c-myc phosphodegrons.
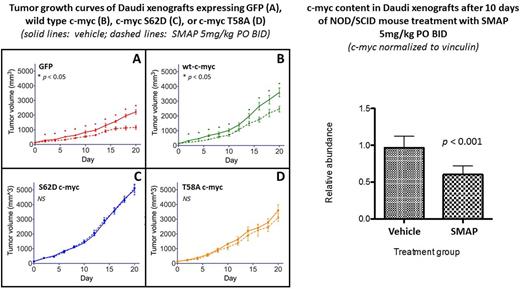
Results: SMAP treatment induced apoptosis in Daudi (BL) cells, as evidenced by decreased viability and increased PARP and caspase cleavage. In Daudi (BL) and CCRF-CEM (T-ALL) cell lines, SMAP treatment led to rapid loss of phosphorylated and total c-myc protein. Cycloheximide (CHX)-chase experiments performed in CCRF-CEM cells confirmed that SMAP treatment affects c-myc degradation without affecting protein synthesis. This effect was abrogated by Ser to Glu mutation of residue 62 within the c-myc phosphodegron, suggesting that SMAP-mediated c-myc degradation is PP2A-dependent. Treatment of NOD/SCID mice bearing Daudi xenografts with twice daily oral SMAP decreased tumor c-myc content and inhibited tumor growth without obvious toxicity. Xenografts overexpressing c-myc with phosphodegron mutations (S62D, T58I) were resistant to SMAP treatment.
Conclusions: These in vitro and in vivo observations establish PP2A activation as a potential pharmacologic strategy to target c-myc in cancers such as BL, T-ALL, and "double hit" non-Hodgkin lymphoma. SMAPs are well-tolerated, orally bioavailable activators of PP2A poised for further exploration in the treatment of c-myc-driven malignancies, either alone or in combination with other agents. Sequencing of the c-myc phosphodegron prior to initiation of treatment may facilitate patient selection in future clinical trials of PP2A activators.
Narla: Mount Sinai School of Medicine: Patents & Royalties: patents on the small molecule PP2A activators (GN).
Author notes
Asterisk with author names denotes non-ASH members.